In the past few years, a lot of people have started having concern about Global Warming. That is excellent. But they seem to all be overlooking a critically important aspect of the problem. We are doing damage that is essentially PERMANENT and it is CUMULATIVE. When spokespeople talk about gradually REDUCING emissions, they are entirely missing the central point. To think about aggressively digging up American coal and American natural gas to reduce dependence on foreign oil supplies sounds brilliant. It has merit for THAT reason. However, such attitudes do not help in the least to preserve the tiny glimmer of hope mankind has for survival, and indeed, actively extinguishes it.
| Year | Total Mass of Carbon Dioxide In the Atmosphere in Metric Tons |
Theor Equil Temp |
|---|---|---|
| 1800 | 2,200,000,000,000 tons | 58ºF |
| 1997 | 2,837,000,000,000 tons | 77ºF |
| 2008.0 | 3,008,000,000,000 tons | 83ºF |
| Right Now |
83ºF | |
| 2108 | 4,605,000,000,000 tons | 127ºF |
You can see that we send another 500 tons or one million pounds of carbon dioxide into the atmosphere EVERY SECOND. And due to our great lust for energy and electricity and comfortable lifestyles, we will not stop until we all die! It is all amazingly simple and obvious!
We will see below that in the 419,000 years prior to the year 1900, there had NEVER been more than 2,300 billion metric tons of carbon dioxide in the Earth's atmosphere. We see here that due to our activities, the number is already tremendously above that amount, and increasing at a frightening rate.
The point here is that, like with the camel's back, the effects are cumulative, and have been for over 200 years, and that we have absolutely no way to reverse them. Worse, our modern societies, including many new countries, seem intent on breaking the camel's back as soon as they can! The discussion below indicates that it WILL happen within about 100 years, where this continual increase will result in all plants to no longer be able to raise enough water from the ground to keep from drying out and wilting, ending the entire food supply for the world. This seems certain to occur at a time when some children today will probably still be alive to starve to death as a result!
And absolutely NOTHING will be done to avoid it happening! Yes, some token efforts will be done to impress Reporters, and to publicly display the results of billions of dollars of Grants that politicians will give away, but any actual significant effort would be extremely detrimental to all modern businesses.
Political leaders seem to assume that IN THE FUTURE they or their replacements will "handle the problem". That therefore allows their friends, the billionaires who run the largest Corporations, to proceed as they wish today in pursuing profits, until some latest possible date. They are wrong. But since they KNOW that no one can absolutely identify such an indisputable date, they know that no one will stop their pursuit of profits! Those political leaders and business leaders KNOW that they can slide by without having to make any "difficult decisions" which would hurt giant Corporations (profits) that their careers are financially dependent on.
The problem coming is that at some point,
the Earth will warm up to a point where PLANTS cannot draw sufficient
water from the soil to keep their leaves from drying out and dying.
Plants happen to be the ONLY actual source of food for people and
animals on Earth. Once all the plants die, there will be no source
of food at all, and all animals and people will necessarily die of
starvation within months of that. (Archer and Barber, 2003, Photosynthesis
and Photoconversion, Chap 1, p.4) |
When will anyone KNOW FOR SURE that all the plants are in the process of dying? Sadly, only after it has proceeded to a point where it can not be stopped. How many straws were involved in the fable about the camel? No one could know, until it happened.
There have been a number of Botanist and Agronomist Researchers who have given numbers that are generally around 80ºF for an average Earth temperature where essentially no plants will then be able to live (compared to today's average of around 58ºF). But they can't PROVE such statements regarding the entire Earth! (Our linked analysis regarding the CURRENT equilibrium temperature for the Earth indicates that it is already around 83ºF).note 35
Businesses and leaders will be able to always deny the very existence any such effects, and any responsibility by them for such effects, until far after it will be too late. There is a valid possibility that that situation HAS ALREADY HAPPENED (due to our past efforts at having dumped around 800 billion tons of carbon dioxide into the atmosphere already by burning fossil fuels.)note 35 note 32 So there is a definite possibility that we are ALREADY beyond the point where human life will be able to continue for very long on Earth!note 32
People seem to insist on knowing SOME EXACT AMOUNT of carbon dioxide, either annually or cumulatively, that we could safely dump into the atmosphere. THAT situation has certainly already passed. The correct answer to that question is ZERO or probably even a NEGATIVE NUMBER! Every single gallon of gasoline or heating oil burned by each of us, simply makes a doubtful situation even worse. Every pound of coal burned to produce the electricity we all feel we cannot live without, the same. Whether there is (1) NO CHANCE; (2) A TINY CHANCE; (3) or A DECENT CHANCE; of some human life being possible on Earth beyond about 100 years from now is a question that cannot be answered. Humanity will only know that answer for sure when it gets there!
But EVERY DAY we are INTENTIONALLY CHOOSING to reduce our odds of survival of mankind even more. We keep adding extra straws to the camel's back. Indeed, we are now shoveling extra straws on in the largest quantities that our modern technology can accomplish! The result is that EACH DAY of what we call "modern society" we are bringing even closer the day when humanity will have self-exterminated.
We are ALL fully responsible! Every gallon of gasoline that YOU burn up in your vehicles sends another 18 pounds of carbon dioxide into the atmospherenote 1, which will likely remain there for hundreds of thousands of years. Every gallon of home heating oil you use, the same. Every lump of coal burned to create the electricity you use, yes.
YOU claim that you only use small amounts, a wonderful cop-out. But there are over six billion of us humans alive, and even though EACH of us only adds moderate amounts, collectively we add astounding amounts to the atmosphere. THIS year, we humans will burn enough coalnote 13, oilnote 14, and natural gasnote 15 to ADD over 400,000,000,000,000 cubic feet of carbon dioxide to the Earth's atmosphere.note 19 Even though the Earth is very large, that enormous amount is significant. And remembering that this is all CUMULATIVE, we will do the same thing NEXT year, and the year after, and the year after that. All that new carbon dioxide does not go away and instead it simply keeps accumulating in the atmosphere, just like the straws on the camel's back. Until we manage to cause a consequence where we all die. Again, there is a valid possibility that has already happened, but we just will not know it for some decades to come. (possibly about 140 years delay, per the calculations and analysis of this link)note 35
And the people, us, who want the convenience of driving automobiles and trucks, can simply claim denial of any responsibility, or even any validity of these matters. And the giant corporations that manufacture and sell such vehicles and the gasoline and diesel they burn up, can talk about YOUR comfort and convenience and thereby avoid having to face their complicity in the end of humanity. It all works out quite conveniently! Since WE will not personally be damaged or die as a result, it is extremely easy for everyone involved to deny everything that is uncomfortable to face!
However, all such spokespeople are being incredibly deceptive to the public, and ALWAYS with the intention of something that involves either NEW giant profits or the maintenance of EXISTING giant profits.
Here is why it is all deception: THIS YEAR, we humans are burning up an enormous amount of coal (about 4.5 billion metric tonnes), petroleum (about 30 billion barrels) and natural gas (around 3 trillion cubic meters). The specifics are in these footnotes, where that fossil fuel burning IS producing around 13.2 billionnote 13; 12.8 billionnote 14; and 5.9 billion metric tonsnote 15, respectively, of carbon dioxide. A total of around 31.9 billion tons of NEW carbon dioxide is being produced and released into the atmosphere THIS YEAR.note 19
NOTE: We will discuss below the fact that the US government chooses to describe these amounts in a peculiar way, by describing an MMTCe (millions of metric tonnes of carbon equivalent) rather than stating the actual amounts of carbon dioxide. That number only counts the CARBON atoms in the gas and not the actual molecules of carbon dioxide! The effect is that when such amounts are described as MMTCe, they are lower numbers, actually 12/44 of the actual amounts of carbon dioxide (the atomic weight of carbon divided by the atomic weight of carbon dioxide). So where we are discussing (worldwide production of) 25 or 30 billion tons of carbon dioxide, the official figures describe these same amounts as around 6,000 to 8,000 MMTCe. The United States currently creates about 1/4 of all of the world's production, so the US contribution is commonly described as around 1,500 to 2,000 MMTCe. note 8
Say that we do not even intend to actually REDUCE the quantity of carbon dioxide in the atmosphere, but rather simply intend to capture and process the amount THAT WE ARE ADDING THAT YEAR. We just saw that this is already over 30 billion tons of carbon dioxide. We will discuss the logistics of this below, but for now just note that a full 18-wheeler semi-tanker-truck can contain and haul around 20 tons (40,000 pounds) of liquid, or slightly less as compressed gas. A simple division of 30 billion tons by 20 tons per load, tells us that we would require 1,500,000,000 full truckloads of carbon dioxide to be captured, processed, pumped, hauled and then stored.
If those spokespeople are talking about ONE full truckload, yes, they might be exactly correct in their statements. It's just that none of them have ever even thought about more than a BILLION FULL TRUCKLOADS EVERY YEAR, which is about FOUR MILLION FULL TRUCKLOADS EVERY DAY, and THAT would only be to deal with what we are ADDING TO the problem and not actually solving anything!
I remember as a child being told of the hypothetical ethical and philosophical dilemma of having the ability of "pressing a button" which would cause some distant person in some jungle to suddenly die but also that you would receive a million dollars. The question was "Would you press the button?" We seem to be in a similar situation today, except that it is NOT some distant stranger, but likely YOUR OWN children and grandchildren that WILL die due to our choices and actions and lifestyles of today.
The following facts and logic suggest that there is a VERY good chance that EACH YEAR of our CURRENT prosperity of society, very likely will shorten the future of any people being able to live on Earth, by possibly TWO MONTHS for each current year. And this is NOT something that would occur thousands or millions of years from now, but very likely only around ONE CENTURY from now.
For many years, people have known that we are totally dependent on plants for our food supply (even animals and fish we eat were dependent on plants as food.) We HAVE ALREADY CAUSED a situation where the Earth seems (soon?) certain to get so warm that NO plants will be able to draw enough water from the soil to keep themselves from drying out and dying. And in case there might be any doubt about that, we have chosen a course that ensures that we are DAILY making it more and more an unavoidable certainty. By around 2080, no conventional crops will likely be possible in the United States or most of Europe or anywhere in Africa. There is a decent chance that there will be NO trees, crops, or even grass ANYWHERE in Africa or in most of the United States at that time. YOUR CHILDREN will likely witness that effect! But as of the damage we have done so far (2008), there actually COULD be some (limited) locations on Earth where crops, or at least some types of plants, might be successfully grown, in the time scale of even 2150 AD.
The plants in the oceans will be similarly exterminated, but in a more complex manner. Ocean waters are already getting much increased levels of carbon dioxide in them, which is creating massive increases of carbonic acid being formed in the oceans. That acid tends to dissolve the shells of tiny ocean plants, dissolve coral reefs and decrease the pH of the ocean waters (acidify) where plants will have great trouble surviving.
People do not seem to realize how aggressively we are changing the climates on Earth, just by living what we think are comfortable modern lives! Since human nature will cause virtually all people and businesses and governments to insist on maintaining and improving their current economies and lifestyles, it is certain that we WILL shorten that time scale down to probably around 2090 when the Earth will have gotten so warm that even all cactus will die. Within months of that end of the food supply, all remaining animals and people will starve to death. If this actually does happen around 2090, then children living today will be some of those last humans to die. (Archer and Barber, 2003, Photosynthesis and Photoconversion, Chap 1, p.4, calculated those things several years ago, and expressed their concern that the end of human and animal life may soon happen.)
Is it worth us having six years of the comfort and prosperity of what we call modern society, if that is very likely to shorten the lives of your own children and grandchildren (and all other humans) by a year? An interesting question to ponder.
| . |
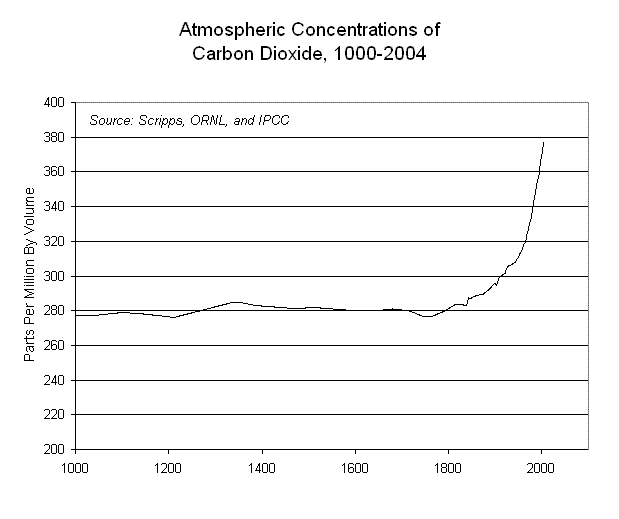
It is obvious that before the Industrial Revolution began (around 1800), the concentration of carbon dioxide in the atmosphere was fairly constant. The ice core data graph below extends that relatively constant line back for a VERY long time (compressed here, but around 420 computer screens to the left), where it was NEVER above 300 ppmv. SINCE the Industrial Revolution began, and we started burning a lot of fossil fuels (coal, oil and natural gas), the curve goes wildly upward, and WILL cross through where the red blotch is far above the existing graph.
We will often abbreviate "parts per million" of the total atmosphere as ppm. This chart shows that the concentration of carbon dioxide was moderately constant at around 280 ppm for the 800 years from 1000 AD to 1800 AD.
It turns out that when snowflakes fell long ago and then became compressed into ice, tiny bubbles of the air (between the snowflakes) of that time got trapped in the ice. Many Research projects have been done (mostly in Antarctica and in Greenland) regarding examining (drilled) ice cores from as much as ten thousand feet deep of ice. This research has provided ACTUAL AIR from the past 419,000 years. This ice-core data therefore extends the Scripps graph back a long way. Think of the Scripps graph being extended to the left around 420 computer screens, with the line never varying much from what we see in those 800 years. In fact, that data shows that there was NEVER any time when the concentration was above 300 ppm (or below about 182 ppm) during the past 419,000 years, Here is the published data (in Nature, June 3, 1999) from the highly respected Vostok ice core research:
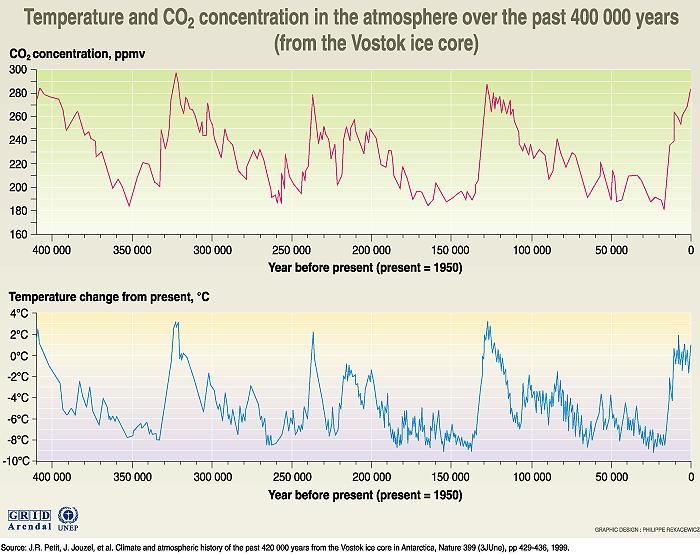
In other words, there is NO DATA in Earth's history for what might happen if and when the concentration would rise above 300 ppm. Remembering that, look again at the right hand side of the Scripps graph, where the curve goes so rapidly above 300 ppmv. The BEST we can do is to simply GUESS at what the atmosphere's response will be to the 300 ppmv of 1900. Or the 370 ppmv of 2000. Or the 586 ppmv which will exist in the year 2100 AD. We seem to have decided to "play the Lottery" in hopes of "winning" which means surviving. The odds against us seem to be comparable to the odds of the Lottery. And no one seems to really care!
We note one additional fact from this pair of graphs from the highly respected research. The temperature graph follows the carbon dioxide graph moderately well as to shape, but note that whenever there was a change of around 100 ppm, either up or down, in the upper graph, there was around a corresponding 10ºC (or 18ºF) change in the Earth's average temperature. This is important in the related Global Warming presentation because we are recently looking at over a 100 ppm increase (so far) in CO2 concentration (from around 280 to 390 ppm). The data from these Vostok graphs seems to indicate that we should therefore expect at least a 10ºC or 18ºF rise, which is in fairly good agreement with the 25ºF rise calculated in the Global Warming presentation's logic.note 35 If the resulting average Earth temperature is therefore 76ºF or 83ºF, either will very likely be terminal to the Earth's plant life.
That is important because MANY botanists and agronomists have said that if the Earth ever gets up to such temperatures, then all plants will die. There are many different opinions, but many of those experts say that an increase of even 10ºC (or 18ºF) in the Earth's average temperature will end all plant life on Earth. Not "immediately" but certainly within a few generations of us at most.
Using the (annual averaged) measured South Pole data from US government documents, we can therefore present the following table:
| Year | South Pole Concentration CO2 (ppm) |
Increase (ppm) |
Atmosphere Increase Billions Metric Tons Carbon Dioxide |
Total Mass of Carbon Dioxide in the Earth's Atmosphere Billions Metric Tons |
|---|---|---|---|---|
| 1800 | (est) 280 | av 1800-1900: 0.14 |
av 1800-1900: 1.1 |
2200 |
| 1900 | (est) 296 | av 1900-1910: 0.35 |
av 1900-1910: 2.7 |
2324 |
| . | ||||
| 1958 | 314.78 | - | - | 2472 |
| 1959 | 315.64 | 0.86 | 6.70 | 2479 |
| 1960 | 316.45 | 0.81 | 6.31 | 2485 |
| 1961 | 317.08 | 0.63 | 4.91 | 2490 |
| 1962 | 317.62 | 0.54 | 4.21 | 2494 |
| 1963 | 318.35 | 0.73 | 5.69 | 2500 |
| 1964 | 318.68 | 0.33 | 2.57 | 2503 |
| 1965 | 319.42 | 0.74 | 5.77 | 2509 |
| 1966 | 320.72 | 1.30 | 10.13 | 2519 |
| 1967 | 321.32 | 0.60 | 4.68 | 2524 |
| 1968 | 321.91 | 0.59 | 4.60 | 2529 |
| 1969 | 323.12 | 1.21 | 9.43 | 2538 |
| 1970 | 324.32 | 1.20 | 9.35 | 2547 |
| 1971 | 325.12 | 0.80 | 6.23 | 2553 |
| 1972 | 326.00 | 0.88 | 6.86 | 2560 |
| 1973 | 327.62 | 1.62 | 12.62 | 2573 |
| 1974 | 328.49 | 0.87 | 6.78 | 2580 |
| 1975 | 329.50 | 1.01 | 7.87 | 2588 |
| 1976 | 330.60 | 1.10 | 8.57 | 2597 |
| 1977 | 332.03 | 1.43 | 11.14 | 2608 |
| 1978 | 333.69 | 1.66 | 12.94 | 2621 |
| 1979 | 335.03 | 1.34 | 10.44 | 2631 |
| 1980 | 336.98 | 1.95 | 15.20 | 2646 |
| 1981 | 338.26 | 1.28 | 9.97 | 2656 |
| 1982 | 339.39 | 1.13 | 8.81 | 2665 |
| 1983 | 341.17 | 1.78 | 13.87 | 2679 |
| 1984 | 342.58 | 1.41 | 10.99 | 2690 |
| 1985 | 343.82 | 1.24 | 9.66 | 2700 |
| 1986 | 345.32 | 1.50 | 11.69 | 2712 |
| 1987 | 346.99 | 1.67 | 13.01 | 2725 |
| 1988 | 348.95 | 1.96 | 15.27 | 2740 |
| 1989 | 350.44 | 1.49 | 11.61 | 2752 |
| 1990 | 351.77 | 1.33 | 10.36 | 2762 |
| 1991 | 353.12 | 1.35 | 10.52 | 2773 |
| 1992 | 354.24 | 1.12 | 8.73 | 2782 |
| 1993 | 355.16 | 0.92 | 7.17 | 2789 |
| 1994 | 356.48 | 1.32 | 10.29 | 2799 |
| 1995 | 358.35 | 1.87 | 14.57 | 2815 |
| 1996 | 359.99 | 1.64 | 12.78 | 2828 |
| 1997 | 361.20 | 1.21 | 9.43 | 2837 |
| 1998 | 363.62 | 2.42 | 18.96 | 2856 |
| 1999 | 365.56 | 1.94 | 15.12 | 2871 |
| 2000 | 366.80 | 1.24 | 9.66 | 2881 |
| 2001 | 368.39 | 1.59 | 12.39 | 2893 |
| 2002 | 370.49 | 2.10 | 16.36 | 2910 |
| 2003 | 372.80 | 2.31 | 18.00 | 2928 |
| 2004 | 374.77 | 1.97 | 15.35 | 2944 |
| 2005 | 376.51 | 1.74 | 13.56 | 2957 |
| 2006 | 378.48 381.74 Mauna Loa |
1.97 | 15.35 | 2973 |
| 2007 | 381 | 2993 | ||
| 2008 | 383 | 3008 | ||
The second column gives the (yearly average of the) ACTUAL MEASURED CO2 concentrations at the South Pole since regular measurements began being recorded there in 1958. The third column gives the DIFFERENCE of the year value with the previous year, that is, the INCREASE in carbon dioxide concentration in the atmosphere.
The fourth column is the ACTUAL AMOUNT OF INCREASE of carbon dioxide in the Earth's, in billions of Metric Tons, during that year. The numbers in this column are based on the following:
The total mass of the Earth's atmosphere is 5.136 * 1015 Metric Tons. This number is extremely well confirmed, and YOU can even confirm it! The "atmospheric pressure" (14.7 pounds per square inch in the English system) is actually simply the total weight of all the air stacked above that square inch, all the way to the top of the atmosphere. So simply multiplying that number by the total area of the Earth's surface (in square inches!), and the product is the total mass (weight) of the entire Earth's atmosphere.
The concentrations of carbon dioxide are specified in PARTS PER MILLION, so we need to take this into account. There is one further complication. The concentrations are measured in VOLUME and not WEIGHT or MASS. (ppmv and not ppmm). Therefore, we need to also take into account that carbon dioxide has a higher density than normal air, by the factor of the molecular weight ratio. Carbon dioxide has an atomic weight of 44. The mixture of nitrogen and oxygen that makes up nearly all of our atmosphere has an atomic weight of around 28.8. This makes the density of carbon dioxide 44/28.8 or 1.529 that of air. Therefore, we applied these two factors to the differential value in column three to calculate the actual number of tons of carbon dioxide that was added to the atmosphere during that previous year, in column four.
The value in column five is the cumulative total of the increases, which is therefore the ACTUAL running total number of billions of tons of carbon dioxide in the entire atmosphere of the Earth. It is obviously also exactly proportional to the column two value of ppmv.
We will shortly discuss the situation where we will continue with similar usage as today for the next 100 years. Realistically, this is extremely conservative, because it does not consider the additional burning of fossil fuels in China or India or many other countries.
| Year | Concentration CO2 (ppm) |
Increase (ppm) |
Atmosphere Increase Billions Metric Tons Carbon Dioxide |
Total Mass of Carbon Dioxide in the Earth's Atmosphere Billions Metric Tons |
|---|---|---|---|---|
| each inter- vening year |
. | 2.0 | 15.70 | |
| 2108 | (est) 586 | 2.0 | 15.70 | 4605 |
We can also see that more recently, far more aggressive usage of not only coal but also petroleum and natural gas has increased not only the height of the graph line but the SLOPE of the curve in the graph, as well, meaning that we now are adding far more than just the one billion tons added each year during the 1800s or the three billion tons added each year in the early 1900s. In fact, this chart shows us that we have been recently adding between FIFTEEN and THIRTY billion tons each year, such that the current concentration is now very close to 390 ppm.
Whether it is intentional deception or accidental choice, the US government decided some years back to describe these amounts in an odd way. See this Footnotenote 9
This is again in excellent agreement with the other figures above, which confirms that they are each very reliable.
That had always been a good thing in the past! Before the Earth had a significant atmosphere, the natural Radiative Equilibrium which existed had necessarily caused the average temperature of the Earth's surface to be around -9ºF or -23ºC.note 35 That was so cold that very few places even had any liquid water, so life was then essentially impossible. It was only after enough carbon dioxide had been released into the atmosphere (on the order of 280 ppm) that the average temperature of the earth had risen to the 13.75ºC or 56.75ºF which was true around 1900 AD.note 35 In general, temperatures fluctuated during the existence of the Earth, as we have seen in the Vostok temperature graph for the past 419,000 years, but they have always been conducive to organic life, for several billion years, never terribly different from that 1900 temperature.
Our human activities, during the past 200 years and especially during the most recent 50 years, of mining truly enormous amounts of coal to produce electricity and even more enormous amounts of petroleum and natural gas removed from deep inside the Earth, have released astounding amounts of ADDITIONAL carbon dioxide into the atmosphere.
The amount in the atmosphere is CUMULATIVE, meaning that each extra gallon of gasoline or heating fuel burned sends around eighteen new pounds of carbon dioxide into the atmosphere that will STAY THERE for thousands or millions of yearsnote 14.
There WAS around 2,300 billion tons of carbon dioxide in the atmosphere around 1900 AD. In the early part of the Twentieth Century, we only burned enough coal, oil and natural gas to add two or three billion NEW tons to it each year. But the usage and consumption of fossil fuels has increased at a frightening rate, where we now are adding 25 to 30 billion tons of carbon dioxide each year. Many countries are now even intending to find ways to send even more carbon dioxide into the atmosphere each year! Drilling under the North Pole for oil. Drilling off the California coast, and all over in and around Alaska. And they will certainly succeed. The political force of uncertain energy supplies seems to trump all other considerations. I guess all those Senators and Congressmen are so old that they do not have young children who will die of starvation due to this matter!
In our small addition to the data chart above, we summed up those (current) amounts for the next hundred years. We used the average of 2002-2006, a number of 15.7 billion extra tons being added each year (while we have seen both 25.29 and 31.9 billion tons confirmed GENERATED for different recent years). Assuming that NO ADDITIONAL ENERGY PRODUCTION is developed over the next 100 years (yeah, right), we simply multiplied that 15.7 billion tons that we are certainly adding each year to the atmosphere lately by the 100 years of the Twenty-First Century, and we see that we WILL add around 1,570 billion NEW tons of carbon dioxide to the atmosphere, so that there will then be around 4,605 billion tons of carbon dioxide in the Earth's atmosphere. (For clarity, since the total mass of the atmosphere does not materially change, the total (cumulative) mass of carbon dioxide is always proportional to the concentration in ppmv. Therefore, the 2,304 billion tons of the year 1900 was a concentration of around 296 ppm, while the 3,035 billion tons of today is a concentration of very nearly 390 ppm. And in one hundred years, 2108, there will be the 4,605 billion tons or 586 ppmv concentration present then. However you choose to describe it, we managed to increase the total amount of carbon dioxide in the atmosphere by about 1/3 during the Twentieth Century, and we are intent on doing far more during the Twenty-First Century. The Earth had never experienced that great of a change or that rapidly, ever before.
We are absolutely insistent on causing even greater changes in this current Century! The CURRENT situation, due to past sins, is quite likely to ensure the extermination of human life within around 150 years.note 35 The fact that we still choose to insist on constantly making the situation worse every day, not only guarantees that result, but guarantees that it will happen even sooner. Our selfishness regarding living a comfortable and prosperous life has probably already ensured the soon end of all human life, but the fact that we humans will NOT "change our ways" and will insist on continuing our modern lifestyles, pretty much will guarantee that YOUR children and grand-children WILL starve to death when all food supplies end as all plants die of dehydration.
Separate from the horrendous situation of INTENTIONALLY causing the end of all mankind, the fact that this will be personally applied to children and grandchildren of today is unspeakable.
But that is NOT likely to ever get that far! As long as we insist on maintaining our modern lifestyles, which includes fossil-fuel powered vehicles (cars, trucks, trains, aircraft, ships, military vehicles) and fossil-fuel heated homes and factories and stores, and fossil-fuel heated industrial processes, we will CONTINUE to add at least the current 25 to 30 billion new tons of carbon dioxide to the atmosphere each year, which we are now doing. EVERY YEAR! We have examined the effects of this "simple continuation of the lifestyle that we have all come to expect and insist on."
Even if somehow China and India do NOT cause increases in the world's consumption of fossil fuels, and all businesses somehow agree to NOT advance their technologies by using even more fuels, we have therefore to expect to add that 15 to 30 billion tons each year for the next 100 years, or a total added of that 1,570 billion tons, by the year 2108, one hundred years from now. (We are noting here that many small children alive today are likely to still be alive in 100 years, so this DIRECTLY affects them, and certainly THEIR children.)
This means that we will increase the current 3,035 billion tons in the atmosphere up to 4,605 billion tons, right? Where 3,000 billion tons represents around 390 ppm of the atmosphere, we have seen that 4,605 billion tons represents around 586 ppm.
Look at the Scripps graph above and try to picture where the point (2100 AD, 586 ppm) would be. It would obviously be FAR above the top of the graph! (the very right edge of that graph represents 2100 AD, and 586 ppm would be about in the text two paragraphs above the graph. We tried to put a red blotch about where it will be. You might notice that the red blotch is right in line with the recent curve of the graph, which confirms this amazing fact.)
REMEMBER that we are considering NO NEW USAGE and simply a CONTINUATION OF EXISTING LIFESTYLES. This is the MOST CONSERVATIVE of realistic possibilities! And STILL our children WILL be living in an atmosphere that has AT LEAST 586 ppm of carbon dioxide in it!
In case the CURRENT Equilibrium temperature of 83ºF was not sufficient to kill off all human and animal life fast enough (in possibly the 140 years), the fact that we INSIST on keeping adding more, every year, is certainly speeding up our own extermination! In the event that the linear model used still applied at such high concentrations, the Equilibrium temperature in 2108 may be greater than 127ºF or 53ºC. Even plants like cactus cannot withstand daytime summer temperatures which may be around 190ºF!
So, just in case the damage that we have ALREADY DONE does not kill us all off quickly enough (maybe 140 years), the fact that "we" would never tolerate ANY "hardships" regarding energy supplies, will nearly certainly speed this all up to DIRECTLY cause children living today to DIE.
Will any giant Corporations STOP all their activities? Not a chance. They ONLY think of PROFITS, PROFITS, PROFITS, and so "if people might be affected in a hundred years, our Accountants really do not care! You have no proof!" Will people ALL be willing to abandon and scrap ALL cars and trucks, and then walk and ride bicycles to work? Yeah, right! Will people scrap their central air-conditioners and fossil-fuel burning furnaces?
NONE of that is going to happen, due to human nature!
This means that we are embarked on a rather short journey, quite possibly well shorter than a hundred years, before there will be no food available anywhere on Earth, and the end of all human and animal life on Earth will necessarily soon follow. YOUNG CHILDREN TODAY will likely see it all end! (and die of starvation as a result.)
IF somehow ALL fossil fuel burning ended today, we have already seen that there is the strong likelihood that the average Earth temperature in 140 years will be too high for nearly any plants to survive, and therefore the entire supply of food will end. And THAT is due to an Equilibrium Temperature that would be around 83ºF.note 35 However, since our insistence on maintaining modern life styles cannot be overcome, the very fact that we WILL add at least another 1,570 billion tons of carbon dioxide into the atmosphere during the next hundred years will have the effect of constantly INCREASING that Equilibrium Temperature even more. Whether it rises to 127ºF may be uncertain, but each degree it rises simply speeds up the time when no plants will be able to endure. A concentration of 586 ppm that seems certain in 2108 is DOUBLE what the Earth had generally experienced during the previous billions of years!
In any case, the 140 year delay would still likely apply, to actually get all the way up to the Equilibrium Temperature, but the key factor is actually whenever the Average Earth Temperature rises above a point where plants can no longer survive. Quite a number of Botanists and Agronomists have stated that if the Earth's average temperature ever rises above 80ºF, that virtually no plants will then be able to live. So the question becomes, IF we are embarked on a trip where the Average Earth Temperature may approach 127ºF in around 140 years, how long will it be until it crosses around 80ºF? Nothing else will matter after that! Once all plants die, and there is no more food, then the Chapter of Earth's history that is centered on human activities will be closed.
There is obviously no precise way to calculate such a thing, as there are a lot of variables which affect the results. But I have attempted to make the best estimates possible regarding those variables, and the results are terrifying. By around 2090, virtually all of Africa and South America will have no vegetation, no animals and no humans, with the possible exception high on some mountains. Southern Asia seems likely to have the same time scale. Parts of Europe, the US, and Australia may still have a very limited amount of plant life around 2100. The very last plants (and animals and people) may be in northern Siberia and Canada, and living on (iceless) Antarctica.
Does this mean that we might as well "live it up" and not worry at all about any of this? By continuing as we have been, and possibly enjoying our last years, maybe human existence on Earth might be shortened to 90 or 80 years from now.
Wow! What a dilemma! To enjoy daily life but always know that the process of doing so is directly causing the end of all human life? To all struggle and suffer in Stone Age lives, knowing that it STILL all will likely end, but a few decades later? I hope that we each somehow get to vote on that one! I truly have no idea of which I would choose!
The difference between the two is that one has absolutely NO chance of survival of human society, and the other MIGHT have a tiny chance of some level of survival.
I also wonder how angry those people will be in 60 or 80 years, KNOWING that they were just waiting for everyone to die, and seeing all the movies of us driving around in 6,000 pound SUVs to go some miles to go buy a fifth of alcohol? They will then KNOW that we ALL are totally responsible for THEIR terrible end. And they will have endless records of movies and photos of the foolish things we now consider NECESSARY! Will such people see any sense in causing a 750,000 pound Boeing 747 airliner to rise to 7 miles high, just because a few hundred people wanted to get to some other city an hour or two earlier? Our pictures will all be on their dartboards! WE "did it" to them! And they probably even PERSONALLY KNEW many of us that did it!
In theory, there is obviously a third possibility. That a few; some; many; most; or all of humanity could somehow manage to get through this. Since plant growth is the central crisis, maybe it might be possible for a very small number of people to live on Antarctica (the ice will all soon be gone there, and it IS a continent, where it might be possible to grow gardens. Most of Africa will likely have noon daytime temperatures above 180ºF, where NOTHING could survive. Most other countries will not be quite as hot, but still too hot for any plants to grow. But maybe some future researcher might find a way to genetically modify some plants into being able to endure daytime temperatures above 160ºF. Maybe some researchers may be able to discover totally artificial food, where we would then never have to even rely on farming or plants for survival. Personally, I doubt it, because such modified plants would involve really massive increases in the water flow through the plants from the soil, to keep leaves from drying out and dying, and how long could any society exist if it was centered on entirely artificial food and nutrient sources?
So I guess I suspect that some "elite communities" of wealthy people will move to Antarctica, and attempt to maintain human existence there. What will they do? Will their millions and billions of dollars then do them any good? It's hard to see. Yes, they would likely survive several more decades after everyone else is dead. Will that be worth their billions of dollars? Hmmmmm.
We are some piece of work, huh? We were provided an amazing planet, with apparently endless supplies of every possible resource. But in around 3,000 years of what we call civilization, and in around 200 years of what we (proudly) brag about regarding industrialization, we have managed to destroy it all, where it may be millions of years before it is again able to support organic life, as it was when we started fouling it up.
President Bush and most others insist on focusing on ENERGY SUPPLIES, no matter what the consequences! He wants to drill thousands of new oil wells in Alaska. All the giant oil companies agree, and they want to also drill oil wells in the Pacific Ocean near California, all over the Gulf of Mexico and countless other places. Russia seems intent on drilling oil wells under the North Pole. Thousands of other locations world-wide for future oil wells are being planned.
The argument is ALWAYS the same, of "finding NEW SUPPLIES of the petroleum and supplies of energy that all of our modern societies are dependent on. That is the ONLY desirable aspect of trying to extract every drop of petroleum from the entire Earth. The US is certainly going to go gung-ho at mining coal at ever increasing rates. Canada has massive amounts of oil-shale that will certainly be mined and processed. Not much is going to deter those efforts, especially just the comments of some Nuclear Physicist!
In an astounding demonstration of ignorance, ALL people today seem to choose to believe that it somehow "goes away", but it actually does not! There IS NO "Carbon Fairy!"
What this means is that EVERY gallon of gasoline you burn in your vehicle, which sends around another 18 NEW pounds of carbon dioxide into the atmosphere, HAS THAT EFFECT WHICH IS ESSENTIALLY PERMANENT.
This is a ONE-WAY STREET! We ADD carbon dioxide to the atmosphere when we burn ANY fossil fuels (petroleum/gasoline/diesel/heating oil, natural gas, or coal), and it NEVER LEAVES.
So the EFFECT is CUMULATIVE in the atmosphere.
The linked article shows calculations that suggest this is likely to happen with the current 390 ppm concentration in around 140 years, around 2150 AD. Humans and animals may no longer be able to survive on Earth after that date, except possibly in small numbers near the South Pole.
But now remember that we humans do not seem satisfied with ONLY increasing the concentration to the current 390 ppm! At CURRENT RATES of fossil fuel burning, we showed the simple and obvious logic above that we WILL have increased the concentration to over 586 ppm IN JUST 100 YEARS!
It is as though we humans are not satisfied with still having maybe 140 years of human existence left, we want to shorten it even more! As long as WE TODAY can live it up!
So, in an astounding LUST for more and more power today, and more and more financial profits for the giant energy corporations, it is as though no one really cares that the small children of today might likely DIE due to the direct consequences. But no one today seems to really CARE about "the future", as long as they have cheap gasoline and endless energy supplies, and as long as there are billion dollar profits for those who provide those needs.
THIS YEAR, we humans are adding around 30 billion tons of carbon dioxide to the atmosphere. How much is that? Well, say that we find some way to REMOVE carbon dioxide from the atmosphere, PROCESS it, COMPRESS it, and pump it into semi-tanker trucks. How many trucks would we need? A tanker truck can contain around 20 tons of liquid and nearly as much highly compressed gas. IF we don't really intend to actually try to REDUCE the concentration but rather simply process the NEW carbon dioxide we are adding, that means we would need (30,000,000,000 / 20 or) 1,500,000,000 full truckloads to even MOVE all that compressed carbon dioxide gas! Of course, 1,500,000,000 truckloads would burn up a LOT of Diesel fuel if they actually needed to haul it any distance! And the compressors to squeeze all that gas, and the giant pumps necessary to move it into the tanker trucks, and all the other equipment required? ALL of those things run on fossil-fuels, and therefore they would each and all be ADDING TO the problem they were trying to solve.
Want to guess at the labor costs of driving 1,500,000,000 truckloads to any destination, and the millions of other people necessary to handle, load and process all the different steps of this activity? Even buying the million trucks and pressurized tanker trailers is a considerable expense, as well as maintaining them.
So the people who get on TV to announce some new method or process of removing carbon dioxide, ARE able to show their ideas in a Laboratory, but they have never actually considered the SCALE of this problem. And these paragraphs have not even considered actually trying to REDUCE the problem but merely processing the NEW carbon dioxide that we insist on creating by burning fossil fuels!
Human nature has always been based on HOPE. Some solution is always expected to turn up. It appears that we have finally encountered a problem that is too large for us to even dream of dealing with, and it WILL result in the end of human life on Earth. And there is not a damn thing that anyone can do to stop it.
These various concepts are UNDESIRABLE to Corporation Executives and world leaders, because they each enable individuals to provide for their own needs, and not be dependent on powerful distant people to make decisions.
If all six billion people on the planet would choose to use these methods, AND if all businesses and corporations would choose to eliminate promotion of and use of fossil fuels, including all coal-fired electric plants (which supply around 51% of all the electricity now used in the USA), in theory, it might be possible to eliminate ADDING MORE carbon dioxide to the atmosphere. Note that would NOT solve our problem, but merely keep it from getting far worse! But we all know that is not going to happen anyway. Yes, a FEW people will choose to use concepts such as these, but they are such a tiny fraction of all the people on Earth, that it would be rather meaningless.
Astoundingly, even if businesses and leaders and citizens really fully comprehended that the end of all human life is at hand, most will not actually do anything, especially if they think that it might be less profitable or less comfortable or more of a bother. So even though a FEW people will realize these matters and try to do something, there are so many people on Earth who will not, that the human adventure on Earth is essentially over. This says something about human nature, doesn't it? Since the consequences are NOT "next week", they are seen as irrelevant and to be ignored. Let the NEXT guy deal with such problems! No need to even THINK about such things!
Since that will NOT happen, then each additional year's 20 or 30 billion or more tons of carbon dioxide emissions just drives more nails into the coffin of humanity. The 1% possible chance will constantly get smaller and smaller each year. And we will merrily bop along, each claiming that WE are too tiny to cause any harm, so we will each continue as always. It is really amazing.
There has been virtually no research done regarding how much carbon dioxide is in the deep oceans, or what the possible implications might be if we tried to change it very much. A FEW experiments have been tried (beginning around 1999) but they have generally been failures. Those experiments attempted to bubble carbon dioxide up through ocean water, expecting that the carbon dioxide in the bubbles would dissolve into the water as the bubbles rose. That only partially occurred. Shortly after that process would begin, they discovered that a "rind" formed around each bubble, which then separated the carbon dioxide inside the bubble from the ocean water outside it.
However, the general concept is still very important. And some scientists, even without any facts or evidence at all, choose to believe that MOST of the carbon dioxide that humans are putting into the air will quickly get dissolved into the oceans. IF they turn out to be right, then we have NO problems at all! At least the way that some of the scientists describe it! But keep in mind that there is NO experimental evidence to support their line of logic. In fact, there are TWO lines of logic that seem to completely disable their optimism. (1) One is that the oceans have been around for around four billion years, and they certainly have gotten to some equilibrium condition between the atmosphere and the oceans regarding concentration of carbon dioxide. Yes, as we are increasing the amount in the atmosphere, we are affecting that equilibrium, and certainly are causing SOME additional carbon dioxide to be being absorbed into the oceans. But no one had any way of knowing whether that pursuit of equilibrium will take ten years or a hundred thousand years. And reasonable assumptions in the calculations suggest that the total amount of added dissolved carbon dioxide may be relatively minimal. A few paragraphs down, there is a discussion on the likely MASSIVE RELEASE of carbon dioxide from the oceans as the waters warm even a single degree, and that effect seems to entirely overwhelm any possible advantage regarding dissolving more of our contributions to the atmosphere.
(2) Is even more logically compelling. When we carefully examine the two graphs of the Vostok data, we see that many times in the past 420,000 years, the carbon dioxide concentration in the atmosphere behaved as it should with MINIMAL effect of added or reduced solution in the oceans. That data and those graphs seem to imply that the very optimistic views of those scientists who want to believe that the oceans will simply dissolve all of our errors, are probably not correct.
There is a third reason as well. MOST of the water in the oceans is rather deep. It has been found that that very deep water seems to travel rather slowly. Water that had been near Antarctica, which had become extremely cold, then sank to the bottom of the main oceans (due to density differences) and that very cold water is now SLOWLY making its way northward! This results in extremely COLD water at the very bottom of the Pacific Ocean, even relatively near the Equator! The important point here is that the flow rates seem to be so slow that the TIME SCALE for very much carbon dioxide being able to (somehow) become dissolved, figures to be many thousands of years, because it will be that long before that water ever warms enough to rise and get near enough to the surface to be able to capture any dissolved carbon dioxide.
I am tempted to wonder if this trending is due to a consistent reduction of available carbon dioxide due to formation of calcium carbonate (limestone), peat, petroleum and the like. If so, that slope, of around 25 ppmv per 400,000 years might give us an estimate of the rate that such processes might be occurring. We know from the analysis above that change of 25 ppmv represents around 190 billion tons of carbon dioxide in the atmosphere. If we assume that all other processes average out over time, that might therefore indicate that 190 billion tons of carbon dioxide became chemically bonded and therefore removed from the Carbon cycle, over the 420,000 years of the data. That might suggest that just under half a million tons of carbon dioxide gets naturally removed from the atmosphere each year.
(If this is valid, then this NATURAL removal of carbon dioxide from the atmosphere is only around 1/60,000 as fast as we are currently ADDING carbon dioxide to the atmosphere!)
There seems that a related similar possibility might exist. The Vostok data seems to show four separate shorter periods of even faster reduction of carbon dioxide, with relatively rapid interruptions of increases in between. If we interpret the slope of the four sections as representing the natural removal of carbon dioxide by those chemical processes, the greater slope indicates faster action. Around 100 ppmv per 170,000 years applies, which is around 9 times as fast as calculated above, which would represent around four million tons of carbon dioxide being removed from the Carbon cycle each year.
The four rather rapid upsurges in carbon dioxide seem to also be a pattern which requires some potential explanation. Whether they are due to astronomic factors such as the changes in the ellipticity of the Earth's orbit around the Sun or many other possible causes might inspire useful science.
We know that carbon dioxide is extremely soluble in water, especially in COLD water. The Handbook of Chemistry and Physics tells us that water at 0ºC can dissolve 1.713 cubic meters of carbon dioxide into each cubic meter of water, an amazing amount.
If that water is instead at 1ºC, just one degree warmer, it can dissolve considerably less, 1.646 cubic meter of carbon dioxide. And if raised to 10ºC (which is 50ºF), far less yet, at 1.194 cubic meter. If raised even further, to 20ºC (which is 68ºF), far less yet, at 0.878, only around HALF what can be contained at 0ºC!
The oceans contain around 1.1 * 1018 cubic meters of water. This is also around 1.1 * 1018 metric tons of water. Therefore, if ALL the ocean waters were at 0ºC, they COULD contain an astounding amount of carbon dioxide in them as dissolved gas, around 1.9 * 1018 cubic meters of carbon dioxide (at STP). Since one cubic meter of carbon dioxide (at STP) is around 1.8 kg, we are talking about 3.6 * 1018 kg or 3.6 * 1015 metric tonnes. For comparison with numbers discussed above, this is 3,600,000 billion tons! Remember that the entire atmosphere currently contains around 3,000 billion tons and that we are adding about 30 billion tons each year. Even the entire Carbon Cycle, of the Photosynthesis processes of all plants on Earth, only remove and replace about 300 billion tons each year.
In other words, the potential capacity for the ocean's waters to contain (and absorb) carbon dioxide from the atmosphere (and therefore from our burning of fossil fuels???) could be a thousand times greater than the capacity of the atmosphere itself! (people have already long known that!)
(By the way, no one actually knows the average temperature of the ocean's waters! It MIGHT seem logical to assume that it is around the 13ºC of the current average temperature of the surface of the Earth. But deep ocean probes seem to nearly always find the water to be near 0ºC, with the accepted explanation being that it is due to water that had sunk near Antarctica and is slowly flowing across the ocean bottoms. What is the AVERAGE temperature of all the oceans' waters? It is something that is yet to be researched! It seems to have recently become a critically important fact to know, and yet we are still ignorant of it!)
However, it has to be assumed that over the history of the Earth, the oceans must have ALREADY absorbed the correct amount of carbon dioxide to already be at or near an equilibrium amount. There MAY or MAY NOT be any additional capacity for absorption of carbon dioxide into the oceans.
The far more interesting, and potentially important, aspect of this is the DIFFERENCE which would occur with CHANGES to the temperature of the oceans! Say that ALL the waters in all the oceans rose by just 1ºC. Each cubic meter of that water would then be able to only contain 1.646 cubic meter of dissolved carbon dioxide rather than 1.713. Therefore, as that water warmed up that one degree in temperature, it would NECESSARILY have to RELEASE the difference of 0.0067 cubic meter of carbon dioxide. That is therefore about 7.5 * 1015 cubic meters of carbon dioxide (at STP) or 13,500 billion metric tons of carbon dioxide which WOULD be released! (In comparison, all of our current burning of fossil fuels in a year is adding only around 1/500 of that amount!)
Please note that these figures refer to the MAXIMUM solubility of carbon dioxide in the oceans. When sufficient data is obtained, we will certainly discover that the ACTUAL amount is less than that.
Putting it another way, if the entire mass of ocean water would rise by about two one-thousandths of a degree C (0.002ºC), and if all that water was already fully saturated with carbon dioxide, the amount of carbon dioxide that would necessarily be released would be about the same as all the damage we are doing today with burning fossil fuels!
The point here is that we REALLY have very little idea what we are doing to this planet! Before five years ago, few people seemed to even give any concern regarding fossil-fuel burning and global warming. If there should now be a factor that is A THOUSAND TIMES LARGER which no one has even ever thought about, then we really are playing with fire with no knowledge of the consequences!
SO! We are beginning to realize that we should worry about many things that had never gotten attention before. But it may turn out that what we should REALLY be concerned about is the average temperature of the oceans' waters! If our activities should raise the entire oceans by even 1ºC, we thereby might cause 7.5 * 1015 cubic meters of carbon dioxide (at STP) to become released from the (fully saturated) waters of the world's oceans, WOW!
Continuing our math (density), that would be around 13.5 * 1015 kg or 13.5 * 1012 tons of carbon dioxide going into the Earth's atmosphere. Given that the entire Earth's atmosphere is 5.136 * 1015 tons, it would seem to cause an increase in the current 390 ppmv concentration up to around 2,200 ppmv!
If we are worried about the effects of the current 390 ppmv or even a coming 586 ppmv in a hundred years, no one will want to even contemplate if the oceans warm up and release so much carbon dioxide as to cause 2,200 ppmv.
There is an important aspect of data needed here that I have not been able to find any source of! Is the ocean water currently SATURATED with carbon dioxide, or if not, to what level of saturation currently exists? Again, the long age of the Earth seems to ensure that an equilibrium must have been established far before now. Different researchers seem to express amazingly different opinions regarding how much carbon dioxide is in the deep oceans! This information is important in order to try to predict how much future carbon dioxide will be able to be absorbed into the oceans. It is critical that we set up many research projects to learn what the real values are.
So, for example, in 1998, official reports describe that the US sent 1,494.0 MMTCe of carbon dioxide into the atmosphere. We therefore need to multiply this value by 3.667 to get 5.498 billion tons of actual carbon dioxide that the US emitted that year. Since official documents indicate that was 24.3% of the world total, we can then see that the entire world emitted 22.63 billion tons of carbon dioxide that year.
You might have noted that there was only a 19.48 billion tons increase in the atmosphere that year. Where did the other three billion tons go to? It turns out that carbon dioxide is extremely soluble in water, and that year, it was absorbed into the oceans. This brings up another important matter, the fact that the solubility of carbon dioxide in water is EXTREMELY dependent on the temperature of the water, with the greatest solubility being in very cold water. When the temperature of the water changes by just a single degree (C), the solubility can change by around 5%. This means that even rather small changes in ocean temperatures can cause enormous amounts of extra carbon dioxide to be absorbed in the oceans or released from the oceans! Things like El nino and La nina change the temperature of large areas of ocean, which seems certain to have huge secondary effects on the carbon dioxide balance. I have never seen any researchers yet address this issue.
So there is yet another dreadful consequence of the Earth rising even one degree C in temperature, that massive ADDITIONAL carbon dioxide will then be released from the ocean waters, compounding the entire problem even more!
We determined in the Nomenclature Footnote above that the US emitted a total of 5.498 billion tons of actual carbon dioxide in 1998. There were then around 300 million of us in the United States, so we might fairly say that we each caused the emission of around 18.33 tons of carbon dioxide, so one might say that each American (including little babies!) had a Carbon Footprint of over 18 tons in 1998. However, it is common to be charitable and separate off the Industrial contribution and also the amounts created by the large trucks and airliners, so it seems common to say that each American has a Carbon Footprint of around 10 tons each year. You can see that there is quite a range of numbers which can correctly be applied here. Also, I am not so sure that tiny babies should be blamed for this, and it might be more correct to describe HOUSEHOLD FOOTPRINT for the 80 million families in the US. In that case, the appropriate number might be around 70 tons, or a charitable 40 tons per family.
There are also others who ignore the carbon dioxide and instead talk about the (somewhat hypothetical) MMTCe number. That number does not even refer to any real chemical, but instead tries to use the quantity of Elemental carbon that is present. We mentioned that, in 1998, official reports describe that the US sent 1,494.0 MMTCe of carbon dioxide into the atmosphere. This would be 1,494 million metric tons which we might try to allocate among the 300 million of us that then were Americans. Dividing, we might then say that each American had a Carbon Footprint of 1,494/300 or 5 tons of carbon each. However, the reality is no different! A family with two children would still be blamed for 4 * 5 or 20 tons of elemental carbon, or the equivalent of 20 * (44/12) 73 actual tons of carbon dioxide, each year.
These comments are meant to confirm that the figures for numbers of tons added to the atmosphere in the table above are accurate, as they agree with other ways used to describe our CO2 emissions.
A pound of Coal therefore contains very close to 0.8 pound of carbon in it. If it is burned extremely completely, we can assume that ALL that carbon will combine with oxygen from the air to form carbon dioxide. Using atomic weights again, we see that carbon dioxide is 12 + 16 + 16 or 44, since oxygen is 16. When the 12 weights of carbon is burned (oxidized), it therefore forms 44 weights of carbon dioxide. We had 4/5 pound of carbon to start with so we multiply 4/5 * 44/12 to get 44/15 or 2.93 pound of carbon dioxide formed for each pound of Coal burned.
We can examine the official Reports for any year, regarding the CONSUMPTION of Coal in that year. Such Reports tell us that 2.148 * 109 metric tons of oil equivalent of coal was burned in the year 2000 (worldwide). Such Reports give oil-equivalent numbers, because different kinds of coal have rather different energy contents. If we take oil to contain around 19,500 Btus per pound and an average coal to contain around 13,000 Btus per pound, we then have to multiply by 1.5 (or 19,500/13,000) to get the actual amount of tons of coal burned. Therefore we have 3.22 * 109 metric tons of coal burned in 2000.
We just determined that each pound of that coal creates 2.93 pounds of carbon dioxide when it burns. Therefore, in the year 2000, the amount of coal that was burned produced 3.22 * 2.93 * 109 metric tons or 9.44 * 109 metric tons of carbon dioxide.
THIS year, the massive increases in coal burning in China to produce electricity and to power their many factories indicates that at least 4.5 * 109 metric tons of coal is being burned, which is creating about 13.2 * 109 metric tons of carbon dioxide.
Consider starting with two pounds of coal, which we just discussed contains 2 * 13,000 Btus of chemical energy in it, 26,000 Btus total.
As the two pounds of coal is burned, we learned above that 2.93 * 2 pounds or 5.86 pounds of carbon dioxide is formed.
It is not possible to burn coal with perfect efficiency, and it is also not possible to transfer all the heat created into forming steam from water. The mechanisms of the steam turbine and the electrical and magnetic fields of the alternator are also not pf perfect efficiency. The net effect of all of this is that roughly 30% of the original energy in the coal is converted into actual electricity. (Nuclear powered plants are slightly more efficient, at around 32%, and fuel oil powere and natural gas powered plants are slightly less efficient, generally around 28% or 29%.) Much of the remaining 70% is INTENTIONALLY THROWN AWAY by cooling towers or equivalent equipment.
In any event, we now have 30% of the 26,000 Btus from our two pounds of coal as actual electricity, or 7,800 Btus. This electricity then has to go through transformers to raise its voltage up high enough to be reasonably efficient in high-voltage transmission lines. It then is sent through such high-tension wires. The standard design rules are to design such lines so that 90% of the electricity put in one end of a 60-mile long stretch will come out the other end. Ten percent of the electricity is therefore lost as resistance heating by the wires, in every sixty miles of such lines. Once in a city, more transformers are used to lower the voltage to around 12,000 volts, for the lines that are up and down every street on utility poles. Then there is another transformer near your house that lowers that voltage even more to the 240 volts and 120 volts that you actually use in your house.
It turns out that all those transformers and especially all those wires have quite a bit of losses in them. For an AVERAGE home at an AVERAGE distance from an electric powerplant, roughly 60% of the electricity put in the wires at the powerplant gets wasted as resistance heating and magnetic losses, so only around 40% of that electricity put in the wires actually gets to our houses!
The OVERALL efficiency of the entire coal-fired electricity generation and distribution system is therefore 30% * 40% or around 12%! Thirteen percent is a more common value, really a disappointingly low percentage!
Since we are tracking the electricity from our two pounds of coal, we now find that only around 7,800 * 40% or 3,120 Btus of electricity actually gets to our house! And since 3,412 Btus is equal to one kiloWatt-hour, we have essentially one kWh of electricity available from the two pounds of coal that was burned up in that distant coal-fired powerplant. Saying this another way, for every kiloWatt-hour of electricity that you use up, there is about 5.86 pounds of carbon dioxide that gets added to the atmosphere.
If your own monthly electric bill shows usage of 500 kWh, that means that you are RESPONSIBLE FOR 500 * 5.86 or 2930 pounds of carbon dioxide that months, about a ton and a half. In the year, that is around 18 tons of carbon dioxide. (It is not usually counted in the Carbon Footprint estimates!)
This burning of coal to produce electricity is the primary reason that coal is consumed in the US, so it accounts for most of the annual totals discussed above regarding coal burning.
We can use the information we just learned to find how much carbon dioxide that an electric powerplant releases in order to DUPLICATE the power in one gallon of gasoline. There are actually two different ways we can do this. (1) We know that a gallon of gasoline contains around 126,000 Btus of chemical energy in it. We just determined that two pounds of coal burned in an electric plant can be expected to provide around 3,120 Btus of electric power at our home. We can therefore easily see that 126,000 / 3,120 or about 40.4 groups of two-pounds of coal would need to be burned to duplicate the actual energy in a gallon of gasoline, around 81 pounds of coal! We can also see that 40.4 groups of 5.86 pounds of carbon dioxide would be released from the coal, or 473 pounds of carbon dioxide! A TERRIBLE situation!
However, there is a more "fair" way to do such a comparison. Internal combustion engines are terribly inefficient, with most modern vehicles having engines that are around 21% (thermally) efficient. That is, of the actual chemical energy in the gasoline, only around 21% of it actually winds up able to move the vehicle. This is commonly described by saying that the EFFECTIVE energy in a gallon of gasoline is 126,000 * 21% or around 26,000 Btus. We can do the same calculations we just did in the previous paragraph, to find that about 17 pounds of coal has to be burned at the powerplant, which releases about 49.7 pounds of carbon dioxide into the atmosphere from the coal.
It might be remembered that when an actual gallon of gasoline was burned, around 18.3 pounds of carbon dioxide is released into the atmosphere. If, instead, either a battery-powered or hydrogen-powered vehicle was used, we see that 49.7 pounds of carbon dioxide has to be released from the distant electric powerplant in order to provide the necessary electricity! Actually, more than that, at least 100 pounds has to be released, because our calculations did not take into account the inefficiencies of the battery charger, the chemical inefficiencies of the battery during charging, the chemical inefficiencies of the battery during discharging, the electric motor used in the vehicle, and the mechanical drive train needed.
So even though all the publicity and the excitement is around battery-powered vehicles being so GREEN, and that future hydrogen-powered vehicles will be the same, the fact that they have to receive their re-charging electricity from distant electric powerplants actually makes them horribly UN-GREEN! Around six to seven times as much carbon dioxide must be released into the atmosphere by any electric powered vehicle than if the vehicle had had a standard gasoline engine! This is not to praise gasoline engines, as they are terribly inefficient! But the public is quite mislead by the people who are aggressively promoting electric vehicles and future hydrogen vehicles! The central claim on which people would be willing to buy such vehicles turns out to NOT be true (because the SOURCE of the electricity is from burning coal. IF the electricity could be gotten from solar or wind or hydroelectric, fine, they would be excellent! We must remember though that 51% of all the huge amount of electricity used in the United States is produced by burning coal.
The fact that the electric powerplant is many miles away seems to be the reason that people feel they can ignore whatever happens there! But it turns out that really bad things regarding carbon dioxide occur any time we want ANY electricity, whether for powering a vehicle or for making toast!
We can examine the official Reports for any year, regarding the CONSUMPTION of Petroleum in that year. Such Reports tell us that 3.54 * 109 metric tons of petroleum in the year 2000 (worldwide). If the Reports give the consumption in barrels instead, 7.33 barrels equals one metric ton. We just determined that each pound of that petroleum creates 3.12 pounds of carbon dioxide when it burns. Therefore, in the year 2000, the amount of petroleum that was burned produced 3.54 * 3.12 * 109 metric tons or 11.04 * 109 metric tons of carbon dioxide.
THIS year, we are burning up around 30 billion barrels of petroleum, which is about 4.1 * 109 metric tons of petroleum, which is creating about 12.8 * 109 metric tons of carbon dioxide.
A pound of Natural Gas therefore contains very close to 3/4 pound of carbon in it. If it is burned extremely completely, we can assume that ALL that carbon will combine with oxygen from the air to form carbon dioxide. Using atomic weights again, we see that carbon dioxide is 12 + 16 + 16 or 44, since oxygen is 16. When the 12 weights of carbon is burned (oxidized), it therefore forms 44 weights of carbon dioxide. We had 3/4 pound of carbon to start with so we multiply 3/4 * 44/12 to get 11/4 or 2.75 pound of carbon dioxide formed for each pound of Natural Gas burned.
We can examine the official Reports for any year, regarding the CONSUMPTION of Natural Gas in that year. Such Reports tell us that 2.438 * 1012 cubic meters of natural gas was burned in the year 2000 (worldwide). We use the density of Natural Gas (Methane) (0.7168 gram/liter) to calculate that this amount is 1.74 * 109 metric tons of Natural Gas. We just determined that each pound of that natural gas creates 2.75 pounds of carbon dioxide when it burns. Therefore, in the year 2000, the amount of natural gas that was burned produced 1.74 * 2.75 * 109 metric tons or 4.81 * 109 metric tons of carbon dioxide.
THIS year, we are burning up about 3 trillion cubic meters of Natural Gas, which is about 2.1 * 109 metric tons of Natural Gas, which is creating about 5.9 * 109 metric tons of carbon dioxide.
(6) CO2 + (6) H2O + energy from sunlight ↔ C6H12O6 + (6) O2
The complex carbohydrate is a chemical called glucose. A wonderful side effect is that oxygen is also given off, which we are then able to breathe!
Plants then use that glucose and chemically convert it into all the thousands of other organic carbohydrate molecules on which all life depends.
In Biochemistry, we know that to form "one mole" of glucose, the plant needs to absorb 686 Kilo-calories of sunlight energy. A mole is the total atomic weight (in grams) of any chemical molecule, so we can add up the 6 Cs (each weight 12) and 12 Hs (each weight 1) and 6 Os (each weight 16), to find that a mole of glucose is 180 grams. We therefore know exactly how much sunlight energy was required to create any amount of new plant material created from the carbon dioxide and water.
Here is a simplified presentation of the basic biochemistry involved. It shows the arrangement of the chemical bonds in the glocose molecule, as well as the actual bond strengths of each of the bonds, which shows the theoretical basis for the 686 kCal of energy that is absorbed from sunlight during photosynthesis and released again during decomposition or respiration.
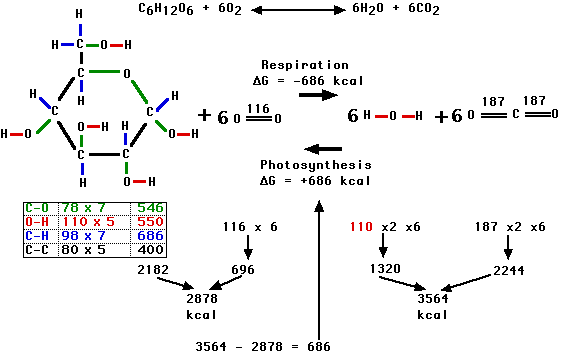
If you add up the total weights of the six carbon dioxide molecules that were used up, you can see that they weigh a total of 264 grams.
The Carbon Cycle is a CYCLE because, when the plants later die, they then naturally decompose (or which also occurs during a common process called Respiration) (with the help of many types of bacteria) back into carbon dioxide and water (or water vapor, the same thing). After an entire Cycle has occurred, the amount of Carbon has not significantly changed.
On the entire Earth, there is roughly 100 billion tons of Carbon involved with the Carbon Cycle each year. We notice that it accounts for 72 (6 * 12) of the weight of the glucose's 180 weight. Since the Carbon Cycle intimately involves the production of glucose, we can therefore know that 180/72 * 100 billion or about 250 billion tons of glucose is produced each year by all the world's plants. In the process, they REMOVE about 264/72 * 100 billion or around 350 billion tons of carbon dioxide from the Earth's atmosphere (and CREATE 192/72 * 100 billion or 260 billion tons of oxygen which we might then breathe!).
So, BRIEFLY, the Carbon Cycle, the total plant life on the Earth, REMOVES a large amount of carbon dioxide from the atmosphere.
However, those plants all eventually die, and when they do, that 250 billion tons of glucose decomposes. The decomposition process then USES UP the 260 billion tons of oxygen again and the glucose decomposes back into the original 350 billion tons of carbon dioxide and the original 150 billion tons of water.
No net advantage or disadvantage occurs regarding amounts of carbon or carbon dioxide or anything else occurs due to the Carbon Cycle. In fact, the exact same weight (mass) of each of the Elements always exists, around 100 billion tons of carbon, 17 billion tons of hydrogen and 400 billion tons of oxygen. The chemical processes of photosynthesis and decomposition just change the appearance as different atoms combine in different molecular combinations.
The entire Carbon Cycle and the entire field of Biochemistry is more complicated than this simplified discussion might indicate. But the basics are all exactly as described here.
The Carbon Cycle therefore RECIRCULATES all the carbon and carbon dioxide that is available, without ever INCREASING the amounts, except briefly by chemically converting the carbon dioxide (gas) into and out of parts of plants. When we burn fossil fuels, it is ENTIRELY different! We are digging up chemicals which are mostly carbon which have been BURIED for many millions of years. That carbon had therefore been OUT of the atmosphere and the Carbon Cycle for those millions of years. The fact that we dig/pump it all up and then BURN it, means that we are doing what is called oxidation:
C + O2 which gives CO2.
This is NEW carbon dioxide which could not have been created except for the fact that we chose to burn the fossil fuels. Once we have created this NEW carbon dioxide, it is essentially around forever (at least millions of years) AND it is now free in the Earth's atmosphere.
Where the Carbon Cycle never increased the total carbon dioxide in the atmosphere (except temporarily), our burning of fossil fuels IS increasing the total carbon dioxide in the atmosphere, on an accumulating quantity and essentially with forever consequences.
The Solvay Process is still used around the world, related to production of salt, glass, soap, detergent and centrally sodium carbonate. It uses salt water (brine) and ammonia and carbon dioxide to produce sodium bicarbonate and ammonium chloride. As carbon dioxide is bubbled up through the ammoniated brine solution, sodium bicarbonate is formed, which is insoluble and which then sinks to the bottom of the tank. When the ammonium chloride is later treated with lime, the ammonia is recovered and can then be put back in the first step of the process. The only requirements are therefore saltwater, carbon dioxide and lime. THAT is the reason the Solvay process might be a credible possibility, that really only seawater and limestone are needed, both of which are available in very large quantities. The carbon dioxide becomes chemically combined in the sodium bicarbonate, which is insoluble and it therefore precipitates (settles) to the bottom. The carbon dioxide is therefore removed from the air.
There are around 70 Solvay Process plants still in operation around the world. Unfortunately, the total amount of carbon dioxide removed from the Earth's atmosphere each year is very tiny when compared to the scale of our problems. All those industrial plants combined only process about 30 million tons of sodium carbonate each year, indicating that only around 15 million tons of carbon dioxide gets removed from the atmosphere each year. For even the Solvay Process to be of a large enough scale, around 2000 times as many Solvay Process plants would be required to process even just the 30 billion tons of carbon dioxide that we are adding to the atmosphere each year. That would require around 140,000 industrial factories each fully operating the Solvay Process.
All of the hundreds of other chemical processes that we know about which can remove carbon dioxide from air, have far less chance of accomplishing the SCALE that would be needed.
We can describe this quantity in several different ways. By applying the density of carbon dioxide (1.977 gram/liter) we can see that a ton of carbon dioxide gas takes up about 1010 cubic meters of volume. Multiplying, we see that we have about 32.3 * 1012 cubic meters of carbon dioxide. That 32 trillion cubic meters is the same as about 1,140 trillion cubic feet!
(These presentations sometimes use a "more conservative" value of 400,000,000,000,000 cubic feet, as a value that is an average over the past twenty years or so.)
There are some people who get on TV and claim that they will simply collect the carbon dioxide and "sequester" it inside the Earth, such as in caves. They have clearly never done the math! If a volume of 1,140 trillion cubic feet were as a sphere (ball), it would be about 40 kilometers in diameter or 25 miles in diameter. It would have a volume of about 7,800 cubic miles! All the known caves in the world only have a total volume of a few cubic miles!
This analysis ONLY even refers to what we do in a SINGLE year, and we will add just as much again next year, and again the year after!
The running total for the Right Now entry does not include the annual effects of plants growing, what is called the Carbon Cycle. Each year, around 300 billion tons of carbon dioxide is removed from the atmosphere by the plants on Earth, in the process called Photosynthesis. That process combines the energy of sunlight, carbon dioxide, and water to create the basic organic material (usually glucose) which is then adapted into all the organic molecules that all plants and animals and humans require for life. Each year, enough plants and animals die, where their components then decompose back into carbon dioxide and water vapor, to return around 300 billion tons of carbon dioxide back into the atmosphere. The net result is that all the plants and animals on Earth BRIEFLY affect the total amount of carbon dioxide in the atmosphere, but that they collectively remove and return essentially the same total amount each year.
This is the US government data for the top of Mauna Loa mountain in Hawaii. It is easy to see the variation that occurs each year, which is primarily due to the Carbon Cycle. The values for the months of an entire year are averaged to get the government's annual values that we have been using. (Note: variations for different locations obviously occur. Plants grow in opposite seasons in the Southern Hemisphere. The range of variation for a location in Kansas would probably be about four times the range seen on Mauna Loa, since far more plants would be much closer to the measuring location, and there is a greater summer/winter difference of plant growth rate in a Temperate climate). (The seasonal variations seen in the Mauna Loa data are generally about 9 ppm. If that applied to the entire world, it would account for a range of around 70 billion tons of carbon dioxide. However, from other sources, it is well established that the Carbon Cycle involves around 300 billion tons of carbon dioxide being removed from and returned to the atmosphere each year. This is a partial reason why locations such as Mauna Loa and the South Pole were selected as data collection points.)
The Right Now running total presented in this presentation does NOT include these effects of the Carbon Cycle, and instead presents an annual average quantity.
The running total presented here also does not include effects of carbon dioxide being absorbed from the atmosphere into the oceans and being released from the oceans back to the atmosphere. Those effects are not yet very well understood, and even though they appear capable of being large effects, the overall effects of dissolving and releasing carbon dioxide from the oceans is here considered to be a consistent action. The calculations in this presentation indicate that the FOSSIL FUELS ACTUALLY BURNED each year account for around 25 to 30 billion tons of new carbon dioxide created, while the ACTUAL MEASURED CONCENTRATION of carbon dioxide in the atmosphere accounts for around 15 to 20 billion tons which actually becomes added to the atmosphere. The implication is that the difference between these numbers must be the amount that gets absorbed into the oceans. The running total number here is based on a fairly conservative value of 15.7 billion tons actually added to the atmosphere each year (the average of the years 2002 through 2006).
It might be noted that there are certain recent years where the increase in the carbon dioxide in the atmosphere was less than usual (such as 1992-1993), which might imply that an especially large amount of carbon dioxide had been absorbed in the oceans that year. There were also years where the increase was more than usual (such as 1979-1980) and clearly more than human burning of fossil fuels could account for, which might imply that carbon dioxide had been RELEASED from the oceans during that year. Further research is needed in these areas.
The Earth gradually developed an atmosphere, and many researchers assume that the average temperature has increased in a LINEAR proportion to the concentration of carbon dioxide in the atmosphere. Prior to the last two centuries, the concentration of carbon dioxide had stayed fairly close to 280 ppm, and the average Earth temperature stayed fairly close to 14ºC or 58ºF.
We noted from the Vostok ice core data graphs discussed in this presentation that the temperature graph follows the carbon dioxide graph moderately well during that 419,000 years as to shape, but also noted that whenever there was a change of around 100 ppm, either up or down, in the upper graph, there was around a corresponding 10ºC (or 18ºF) change in the Earth's average temperature. This appears to be an excellent confirmation of the mathematical analysis of the Equilibrium Temperature of the Earth.
The two Vostok ice core research graphs show many prominent data points that are in fairly close agreement to the assumption that there is a linear relationship.
This is important in the related Global Warming presentation because we are recently looking at over a 100 ppm increase (so far) in CO2 concentration (from around 280 to 390 ppm). The data from these Vostok graphs seems to indicate that we should therefore expect at least a 10ºC or 18ºF rise, which is in fairly good agreement with the 25ºF rise calculated in the Global Warming presentation's logic.note 35 We have included a column in the table which shows the calculated Equilibrium Temperature which SHOULD exist for each year indicated. If the resulting Equilibrium average Earth temperature is now therefore 76ºF or 83ºF, either will very likely be terminal to the Earth's plant life.
That is important because MANY botanists and agronomists have said that if the Earth's Average Temperature ever gets up to around 80ºF, then all plants will die. There are many different opinions, but many of those experts say that an increase of even 10ºC (or 18ºF) in the Earth's average temperature will end all plant life on Earth. Not "immediately" but certainly within a few generations of us at most.
We included a line for the year 1997, because that year has a calculated Equilibrium Temperature of around 25ºC or 77ºF. The implication is that if humanity had stopped all usage of all fossil fuels on Jan 1, 1997, then eventually the Earth would rise to an average Equilibrium Temperature where Botanists think that the Earth's plants would be right on the borderline of either living or drying up and dying. Therefore, a case seems possible to be made that around January 1997 may have been the last date that we could have altered our behavior such that humanity might have been able to continue on Earth.
The current average Earth Temperature is NOT anywhere close to the Theoretical calculated Average Temperature (yet). Actually, only a rather minimal temperature increase has been noted so far. Some people assume that means that there is no problem. They are nearly certainly extremely wrong about that! The linked presentation describes a situation and provides the calculations regarding a lag time of about 140 years in the Earth's Average Temperature catching up to changes in the carbon dioxide concentration.note 35 The lag time appears to be due to the great mass and therefore thermal inertia of the Earth and the poor thermal conductivity of most of the rocks of the Earth's Crust.
Some spokespeople talk about a "tipping point" regarding causing Global Warming of such a level that humanity will not endure. Different spokespeople talk about "we may have ten years before a tipping point" or "we may have fifty years before a tipping point". None of them appear to have any basis for the time scale they mention, it being pretty much an educated guess. THIS presentation and the linked one DO provide a scientific and mathematical basis for the reasoning and the values presented. There seems to be extremely strong reason to believe that January 1997 WAS the "tipping point" regarding the survival of humanity on Earth.
The hope is that mankind would see cause to apply ferocious dedication to try to correct these matters IMMEDIATELY, and then also find some way to somewhat reduce the amount of carbon dioxide in the atmosphere in coming decades, to give humanity a small chance at survival. Human nature seems to suggest that this will not be done, and that we are (already) doomed.
It seems unlikely that the linear relationship would still hold in 2108, so the Earth's Average Temperature may not get up to 127ºF. But that really will not matter, since if all plants will die when the Earth's Average Temperature passes 80ºF, humans and animals would then all die of starvation and not much else will matter.
An attempt has been made regarding this subject to present it in an unusual way. As much as possible of the advanced science aspects have been moved to a number of Footnotes. The hope is that the resulting presentation might be understandable and tolerable to the public, but that the specifics that careful science insists on are still addressed for those who need or want to know deeper information. The Footnotes are also somewhat abbreviated in not including many things that should normally be familiar to those in the related Fields.
This page - - The Most Dreadful of All Possible News
- - is at http://mb-soft.com/public3/disaster.html
This subject presentation was last updated on 08/09/2008
05:37:38
( http://mb-soft.com/public/index.html )
C Johnson, Physicist, Physics Degree from Univ of Chicago